February 24, 2015 — The Prostate Cancer Foundation (PCF) announces 3 new Challenge Awards to support discoveries for the treatment of lethal prostate cancer. PCF Challenge Awards are multi-year awards supporting cross-disciplinary teams of research scientists. These are the first major awards funded by PCF in 2015.
For more information, visit pcf.org/manhood-for-good
In 2014, PCF solicited proposals for the Global Treatment Sciences Network (GTSN) Challenge Awards as part of an initiative to support research on novel medicines, treatment strategies, and standards of care. All projects must focus on research in humans, resulting in a swift, direct impact in the care of millions of prostate cancer patients and their families. With these new awards, PCF now funds 9 research teams working in the field of treatment sciences.
“Our focus in 2015 is to fast-track discoveries that will have immediate clinical relevancy for advanced prostate cancer,” says Jonathan W. Simons, MD, president and CEO of the Prostate Cancer Foundation. “The innovative work conducted by our Challenge Award teams will revolutionize the way advanced prostate cancer is diagnosed and managed. This will improve the outcome for all men with this disease.”
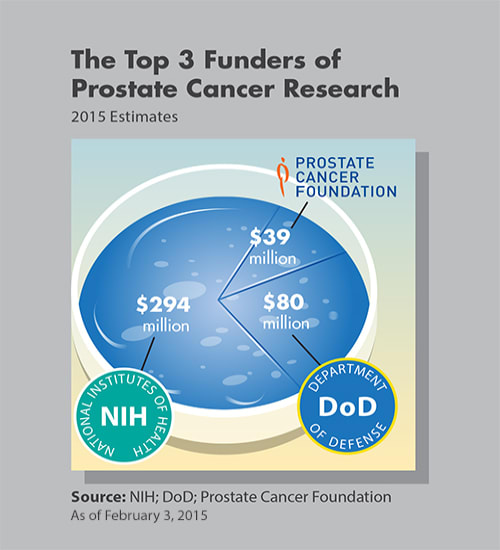
The importance of all 3 PCF Challenge Awards detailed below is their shared goal of putting men with advanced prostate cancer back into longer remission when existing drugs no longer are working. Some men have significant increased survival from prostate cancer treated with chemotherapy; others do not. 2 of the PCF Challenge Awards, however, one from Thomas Jefferson University and the other from Cancer Research UK Beatson Institute will move research forward on correctly predicting which patient is which.
The first of these 2015 PCF GTSN Challenge Awards, titled “Optimizing First Line Treatment for Men with Castrate Resistant Prostate Cancer,” led by William Kevin Kelly, DO, of Thomas Jefferson University, examines whether the status of the retinoblastoma gene in tumors can function as a biomarker to determine whether patients with lethal prostate cancer will respond to abiraterone (Zytiga®) or chemotherapy. As part of this project, Dr. Kelly and his team will develop models from patient data that determine the optimal duration of chemotherapy administration. The results of this work will help clinicians make the best decisions for the treatment of prostate cancer patients.
Hing Leung, MD, PhD of the Cancer Research UK Beatson Institute, leads the second successful project, “Optimizing the Use of Taxane Chemotherapy in Prostate Cancer.” Dr. Leung and team are studying patients treated with chemotherapy in order to identify biomarkers that can predict which patients will most benefit from chemotherapy, and to identify mechanisms of chemotherapy resistance. They will also study how metabolic changes can alter sensitivity to chemotherapy, which will identify novel targets for new experimental drugs that could be combined with chemotherapy.
“T-cell Receptor Gene Therapy for Treatment of Lethal Prostate Cancer,” the third successful proposal, is led by Nobel Prize winner David Baltimore, PhD, of the California Institute of Technology. Dr. Baltimore and his team are studying how patient immune cells (T-cells) recognize prostate tumors in order to generate an effective T-cell gene therapy for prostate cancer patients. They will determine the best strategy for genetically modifying a patient’s own T-cells to fight their tumor and optimize the delivery and efficacy of this therapy in preclinical models. This project will lead immediately to clinical trials, fast-forwarding the delivery of novel therapies to advanced prostate cancer patients.
PCF Challenge Awards are composed of teams of scientists from 3 or more cancer centers. In order to conduct pioneering research, these entrepreneurial scientists require large investments in areas that fall outside the parameters of traditional funding organizations. As a stipulation of funding, all PCF Challenge Award teams must include at least one PCF Young Investigator, demonstrating the Foundation’s commitment to the career development of early and mid-career scientists.
Awardees were selected from a pool of 55 applicants, representing 48 institutions in 13 countries around the world. Each submitted proposal was subjected to a rigorous, two-round peer review process in which the projects were assessed for clinical relevancy and their potential for near-term impact on standard of care. Priority was given to high-risk, first-in-field and currently unfunded projects.
About the Prostate Cancer Foundation
The Prostate Cancer Foundation (PCF) is the world’s leading philanthropic organization funding and accelerating prostate cancer research. Founded in 1993, PCF has raised more than $615 million and provided funding to more than 2,000 research programs at nearly 200 cancer centers and universities. The PCF global research enterprise now extends to 19 countries. PCF advocates for greater awareness of prostate cancer and more efficient investment of governmental research funds for transformational cancer research. Its efforts have helped produce a 20-fold increase in government funding for prostate cancer. More information about PCF can be found at www.pcf.org.









