ASCO 2020: Spotlight on PSMA Therapy Trial Results
Last week, we posted some highlights of PSMA trials presented at the American Society of Clinical Oncology (ASCO) 2020 Virtual Scientific Program. Read on for more details on this novel approach to attacking prostate cancer.
One exciting new area in prostate cancer that continues to be in the spotlight is PSMA-targeted treatment. PCF-funded researcher Professor Michael Hofman, MBBS, of the Peter MacCallum Cancer Centre in Australia has been a leader in this field and serves as the principal investigator of a Phase 2 clinical trial called TheraP. His team’s presentation at ASCO revealed how a PSMA-targeting treatment may represent a potential new class of effective, safe therapy for men with metastatic castration-resistant prostate cancer (mCRPC).
PSMA, short for Prostate-Specific Membrane Antigen, is a protein that is found in relatively larger amounts on the surface of prostate cancer cells. The strategy is to target the cancer cells by creating a small molecule that will bind to the PSMA on their surface. Attached to this molecule is a radioactive element (Lutetium-177, or Lu-177 for short) that delivers radiation directly to the cancer cells, killing them. Thus, the treatment is called Lu-PSMA.
One important aspect of the TheraP trial is that it compares this new PSMA treatment to cabazitaxel – a known active, FDA-approved treatment for prostate cancer – not a placebo! Patients in the trial had resistant disease: they had progressed on docetaxel, and over 90% had been treated with enzalutamide or abiraterone. To be eligible, men also had to have prostate cancer with “high PSMA expression:” the cancer cells had to make enough PSMA to be visible on scans.
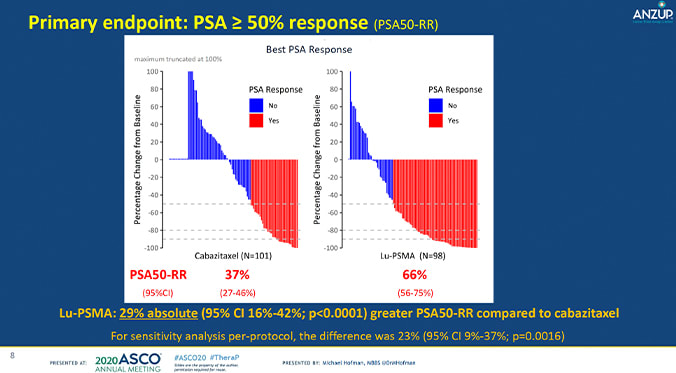
The study divided 200 men into groups, where they received either Lu-PSMA or cabazitaxel. The TheraP study team looked to understand the activity and safety of Lu-PSMA vs cabazitaxel by measuring PSA response, defined as a reduction of PSA of at least 50% from baseline. They also observed PSA-progression free survival (PFS) and adverse events.
In all, there was a large difference in outcomes between the two groups. 66% of participants who underwent Lu-PSMA therapy showed a PSA response, compared to the 37% of participants treated with cabazitaxel. (You can see this visually in the graphic: more red lines in the Lu-PSMA group means that more men responded to this treatment.) This significant 29% percent difference was also accompanied by a lower frequency of Grade 3-4 (more severe) adverse events in the Lu-PSMA group: 35% of patients vs 54% in the cabazitaxel group. These results suggest that Lu-PSMA may be a viable, safe option for men with mCRPC progressing after docetaxel.
TheraP is the first-in-field randomized trial of Lu-PSMA to be completed. The study provided an active control arm, and the substantial difference in PSA response points to a potential novel approach to treating aggressive mCRPC, for which new treatments are urgently needed. The team is continuing to follow other outcomes including quality of life and overall survival.
Prof. Hofman has been a highly prolific PCF-funded investigator, having received a Team Science Challenge Award in 2017. For more on the TheraP trial, click here.










